VRBPAC Presentation for June 5th, 2024
I’m going to jump right into it.
A lot of folks are reasonably concerned about which variant will be picked for the fall vaccine update as this might prevent Novavax availability.
This was a problem back when the Bivalent rolled out, so folks have some reasonable trauma to deal with here…
VRBPAC (Vaccines and Related Biological Products Advisory Committee) is meeting to address updating the vaccine on June 5, 2024.
Let’s get this started with good news.
On Monday, VRBPAC updated its meeting notes to…
“For the 2024-2025 Formula of COVID-19 vaccines in the U.S., does the committee recommend a monovalent JN.1-lineage vaccine composition?”
This is a good sign for everyone concerned about whether Novavax will be allowed to come to market with its JN.1 update.
The FDA is already focusing on JN.1 and that’s not only what will help bring the updated Novavax to market, but it is also the right decision.
I’ll explain, but let’s roll back the clock a bit.
Last year was similar but different.
If you were around last year, we had large scale letter writing campaigns to help with Novavax approval. We tracked down the academic emails for the committee members and contacted them there.
So, you could say we got their attention.
We did it for both VRBPAC (FDA) and ACIP (CDC).
A member of ACIP (Advisory Committee on Immunization Practices) had some choice words for us after our activism…
Here’s the presentation we made for the June 15, 2023 meeting.
Then after that we made a presentation on influenza vaccines on March 5, 2024.
We didn’t think it necessary to do a large-scale letter writing campaign after we were informed by one of the VRBPAC reps that our presentation from June 15, 2023 that focused on Novavax was requested by a few committee members.
So, on June 5th, 2024, I’ll have four minutes to deliver our next presentation and that’s what we’re going to walk through here…
It should help everyone understand the situation better.
Quick point – the links on the screenshots won’t work, but if you want to check out the sources you can find the actual presentation here…
Let’s see if we can answer your questions.
Disclaimer: We are not associated with Novavax, the FDA, or any other group.
Three of us prepared this presentation over three days.
If you like what you see here, please consider making a contribution via Paypal.
Addressing the demands of the moment.
Some frames will need more explanation than others…
But this first one is just a general explanation of what problems the vaccine strategy is facing with a focus on low uptake.
Uptake of COVID vaccines is very low and has dropped even lower since the infographic on the right was made.
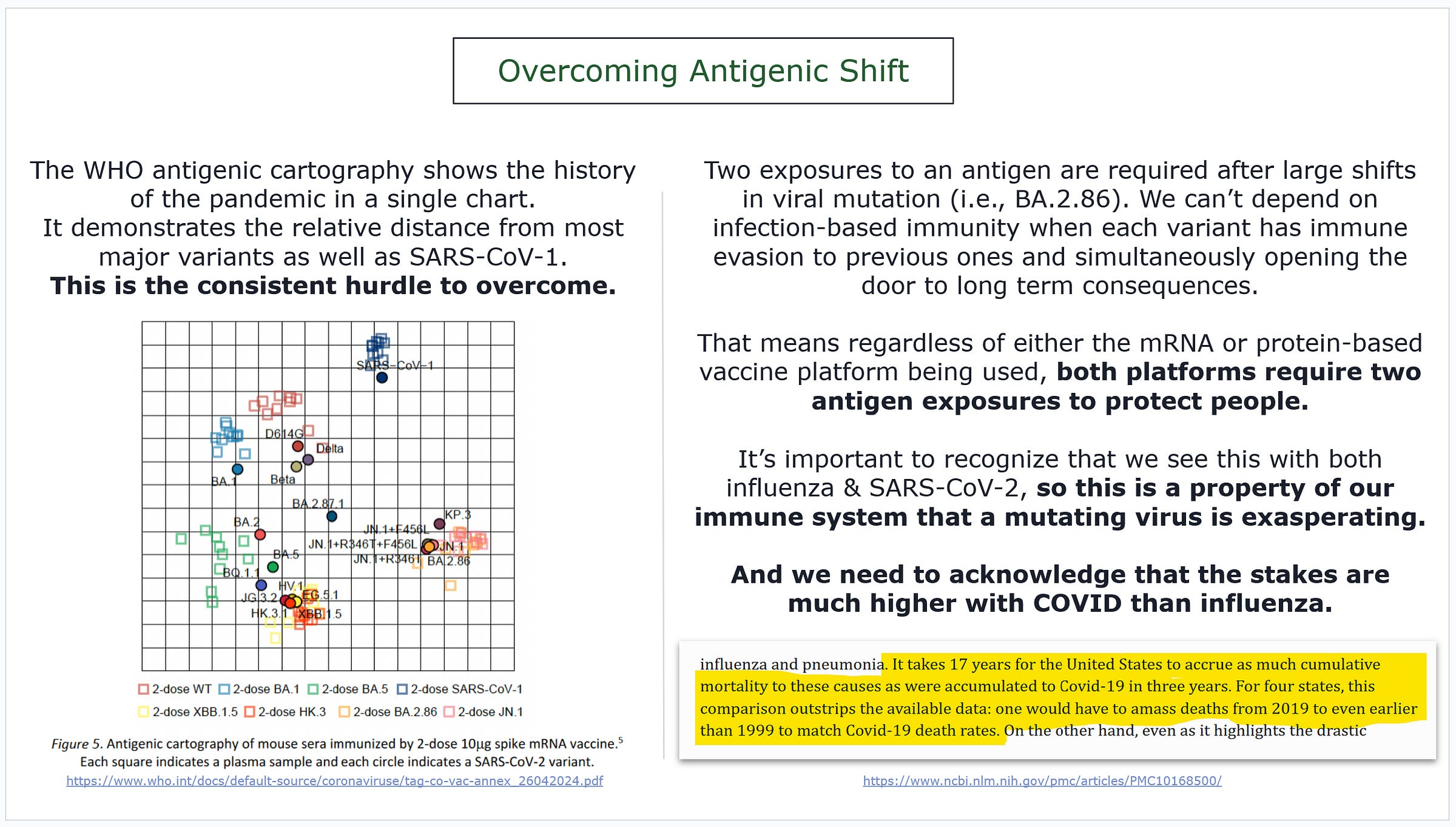
BA.2.86 shifted our antigenic landscape.
It’s hard to describe how dominant BA.2.86 has become compared to other variants that were circulating this time last year… but the chart helps.
“The serological distance between BA.2.86 and wild-type SARS-CoV-2 was found to be longer than from SARS-CoV-1.”
Don’t worry, we have another chart for that later, but this is giving us an idea of how much things changed and gives us our initial focus.
BA.2.86 became JN.1 with just the addition of mutation L455S.
Might as well start throwing darts.
Choosing the dominant subvariant is a total guessing game when only a single mutation can shift the abilities of the virus in such an extreme way.
But we can narrow it down to the JN.1 family with the more modern KP.2 & 3.
It’s important to recognize that the KPs are just JN.1.11.1 subvariants.
The target is a car moving at 100 mph.
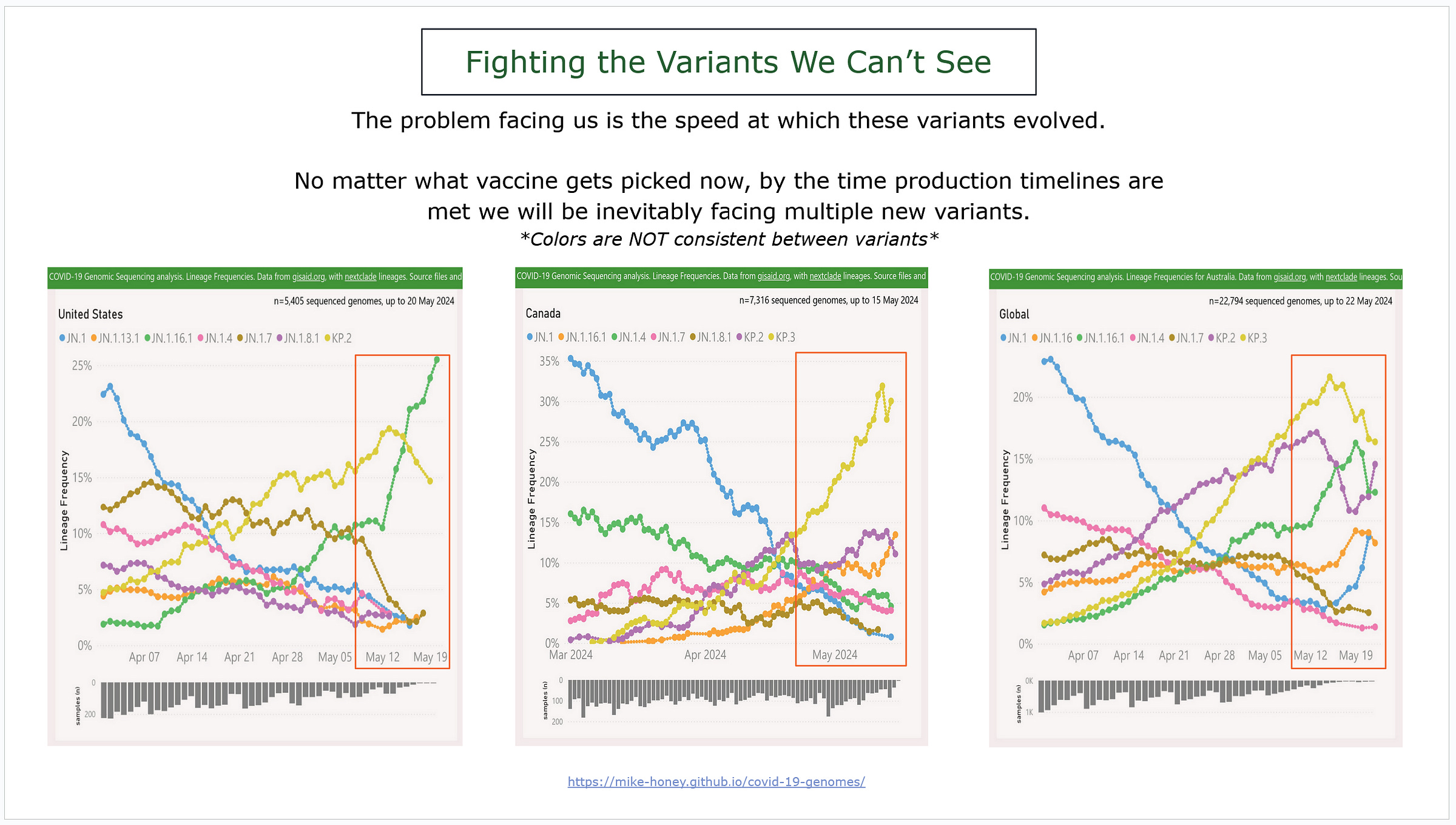
It’s hard to process how quickly these variants went from not even existing to dominant, with some already on the decline.
We’re talking less than TWO MONTHS, some even faster…
As we see a new wave of COVID starting months earlier than we expected then we can easily assume that they have immune evasion to each other.
That means natural immunity is essentially useless and imprinting is a mess.
These charts demonstrate three different examples of variants developing.
Left is the US, middle is Canada, and the far right is the world.
Also important to point out that Canada and the US are sequencing different variants even though they are geographically close to each other.
Naming things shouldn’t be this hard.
When looking at JN.1 and specifically JN.1.11 subvariants KP.2 & KP.3… there are really just two things that come to mind…
These variants have immense immune escape to XBB antibodies.
There are too many dang names for KP.2, making it seem like there are way more variants being an issue than there actually are.
But it’s important to recognize that though they are immune evasive, it is primarily in relation to XBB serology and the immune evasion to each other is not as clear-cut.
We see examples of it, but it’s hard to tell how much is reinfection versus simply new infections and the testing is technically against XBB.
It seems like this poses more of an issue in relation to imprinting than anything.
Someone who might not have been vulnerable to KP.2 might end up vulnerable to KP.3 instead or JN.1.16.1, though we are seeing folks get reinfected much earlier than we expected…
How much of that is immune evasion, immune suppression, or something else is impossible to say without more testing.
But that’s going to leave people with distinct and incomplete memory responses.
Imprinting is an immediate problem.
Part of the influenza presentation was discussing how there is data available showing that folks who get flu vaccines two years in a row with no flu exposure are more likely to be hospitalized.
The connection was not immediately made to imprinting…
But COVID is forcing us into a bit of a corner with this issue.
Imprinting is a good thing; bad imprinting is a bad thing.
At the moment, we don’t have enough finesse to manage it any other way than multiple exposures. But, I think most of us (especially if you are reading this), can agree that depending on infection-based immunity with this many variants is neither ethical nor even a good plan to get the desired outcome.
So, folks need access to a 2 shot regimen over 2 months.
I said we had a chart for that…
The next point actually requires the next TWO slides.
On the bottom left, you will see the antigenic cartography of most of the pandemic, including SARS-CoV-1, as laid out by the WHO.
Note a few things…
distance between BA.1 & XBB
distance between XBB & JN.1
distance between JN.1 subvariants
All distances in that chart are relative to each other.
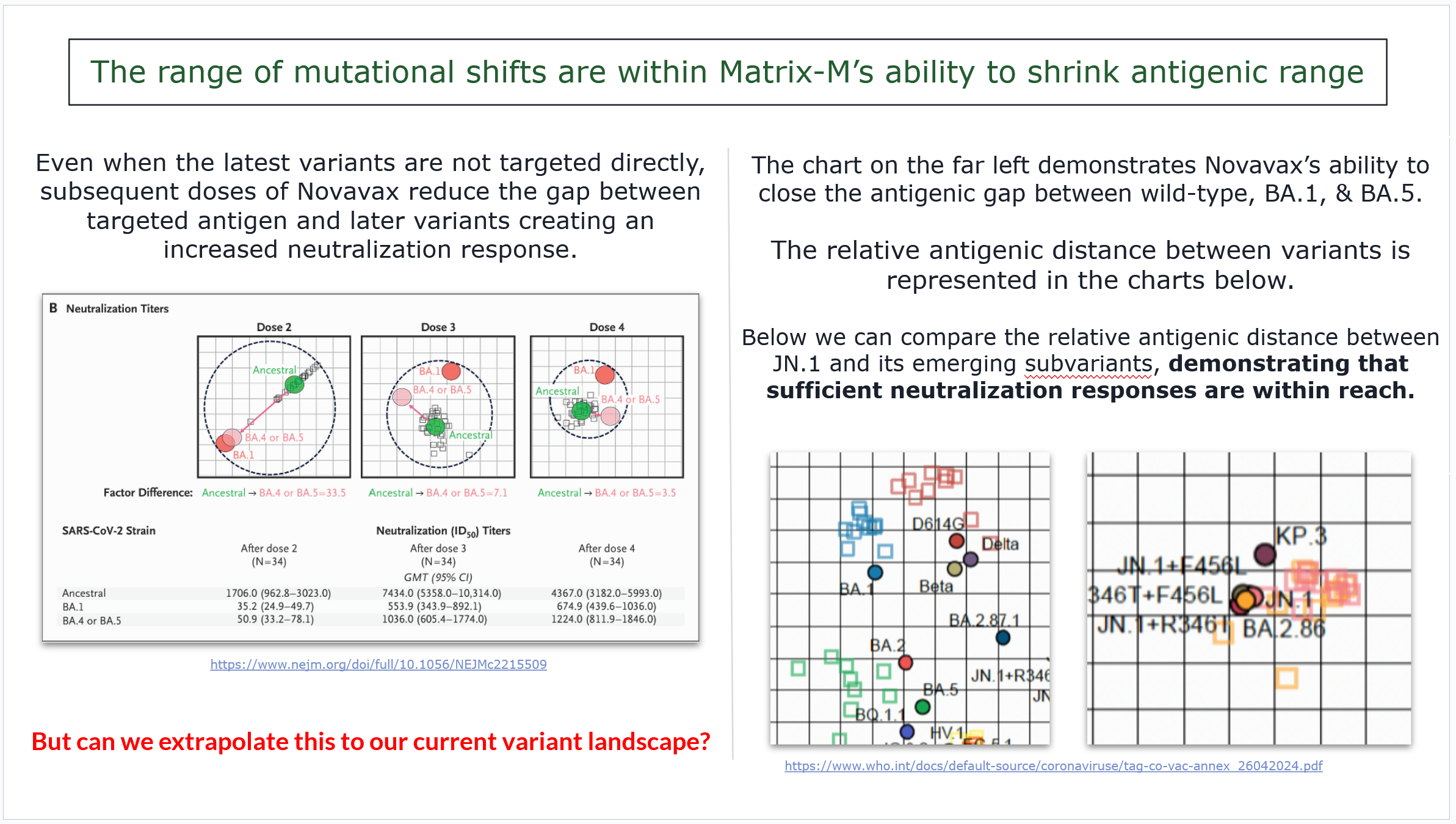
Matrix M can fill the gaps.
Since we have relative distance between omicron variants, we can use Novavax’s charts that show the shrinking of range between those same variants…
And we can then compare the range between JN.1 variants…
This shows us that JN.1 subvariants would be well within the antigenic range of multiple doses of Matrix-M. Since we need two exposures to reset imprinting because of the significance of the antigenic jump, then that’s two problems solved.
JN.1 will be fine, and since we need two exposures for imprinting, the Matrix-M response will fill in that gap with little issue.
Case for targeting JN.1.
This is pretty self-explanatory if you’ve been following the presentation.
We really need everyone to reset their imprinting to JN.1 if they want significant protection from the upcoming wave of circulating variants.
Folks will still get infected, but their immune system will respond faster.
And we know that “JN.1 exposure clearly induces higher neutralization titers against emerging mutants.” That’s when compared to XBB and other sub-lineages that might not be in heavy circulation, but have altered folks’ memory response.
But what about KP.2 and KP.3?
They have sprung up incredibly fast and even switching places with each other at times… But in America, we aren’t seeing KP.3, that’s a prediction…
What we are seeing is JN.1.16.1, at least from genetic testing.
And again, these are ALL JN.1 subvariants.
But back to the speed with which these variants evolved – we can only assume that the next variant is going to hit just as quickly as we continue to move on as if mitigations are not a serious thought.
At this speed, VRBPAC needs to meet twice a year to discuss variants; as that will help our friends in the global south who have winter peak during the northern hemisphere’s summer.
Plus, even when we refer to the chart of JN.1 variants, targeting KP.2 makes more sense than targeting KP.3 because KP.3 stands alone and is already declining in a number of places…
Choosing KP.3 would leave us further out than we want to be from the next major variant that we can’t see yet, which is actually the one we are aiming to create vaccine resistance to.
My personal anticipation is that it will be a JN.1 / XBB subvariant recombinant because XBB subs are still around and persistence is highly prevalent.
Optimal vaccine timing.
I’ll be clearer with this in the oral part of the presentation than I am in the slides, but the idea is to illustrate the most effective timing for Novavax and ensure that it’s available in a stress-free manner.
This isn’t necessarily a topic of discussion for this meeting, but this is the place to make a case for regulatory alterations.
It would be the 2 shots 2 months apart and an additional shot no sooner than six months, but in the 6-9 month window.
It’s important to recognize that this is aiming for peak response. Novavax’s protection from “death and hospitalization” has no clear end point, even without boosters.
But my goal is always focused on reducing Long COVID risk.
IgG4 is still too poorly understood.
I’m not sure how much time I’ll have to address this concept, but IgG4 needs to be worked on more by the FDA, specifically in relationship to pediatric vaccines.
The data on IgG4 and COVID vaccines is full of holes.
We know that the mRNA IgG4 response lasts longer (and that’s not a good thing), while Novavax has the response but has been shown to not be present at six months. Novavax also compensates for the effect with effector and IgG3 responses…
But some data shows that it happens mostly on first exposures and in subsequent exposures it is less prominent.
While we also have data showing that vaccines taken inside of this IgG4 window, specifically from mRNA to Novavax, can show a less than desired response.
As this is a response to new antigen by our immune system, we can only assume this extrapolates to other antigens and vaccines.
Two things…
Until we understand this better, we should limit COVID vaccines to no sooner than six months.
If a child’s first exposure to COVID is from the vaccines, then it might inhibit effectiveness of the traditional childhood vaccines, if they are administered when IgG4 is high.
I’m not suggesting that we expose kids to COVID in advance of being vaccinated. I am saying we need to do more research on how this IgG4 effect might alter the desired vaccine response, and at a minimum, adjust vaccine timing accordingly.
We have already seen occurrences of measles in vaccinated children, a phenomenon which could potentially arise from less than favorable vaccine timing.
Since we are talking about Pediatric vaccines…
Like I said at the start, I am not affiliated with Novavax.
So, I have no idea if Novavax is going to try to push for pediatric expansion for its product. The thing that makes this easy is also the thing that makes this frustrating.
Pediatric Novavax has been proposed and tested as the exact same vaccine that we already have; unlike the overly complicated dosages of mRNA…
It’s all the same shot.
That means this is a matter of expansion of approval, not the approval of an entirely different product.
We’re actually waiting on Novavax to submit their data.
Once I see what they are presenting, then we will pivot appropriately.
Though if we are going to even think about discussing IgG4 and timing surrounding pediatric vaccines, then we should have more vaccines at that table for discussion.
Conclusion.
JN.1 as variant for update.
Allow two shots for imprinting.
Additional shots should be available after six months.
VRBPAC should consider meeting twice a year to discuss variants.
Two shots is required for both platforms, both Novavax and mRNA.
That’s it… Hopefully I can cram that into four minutes of speaking, lol.
If you liked the presentation, you can make a contribution…
We also host a Spaces call twice a week on Tuesday and Thursday.
And we have a COVID-conscious Discord if you’re interested.
The long awaited Novavax timing article should be coming next assuming the JN.1 update will be approved and we know the product will be on shelves.
Though, I did cover most of the information in this presentation.
Anyway, I hope this all helped you understand it a bit better…
Or at least that it didn’t make it more confusing.
…
I should be done here.
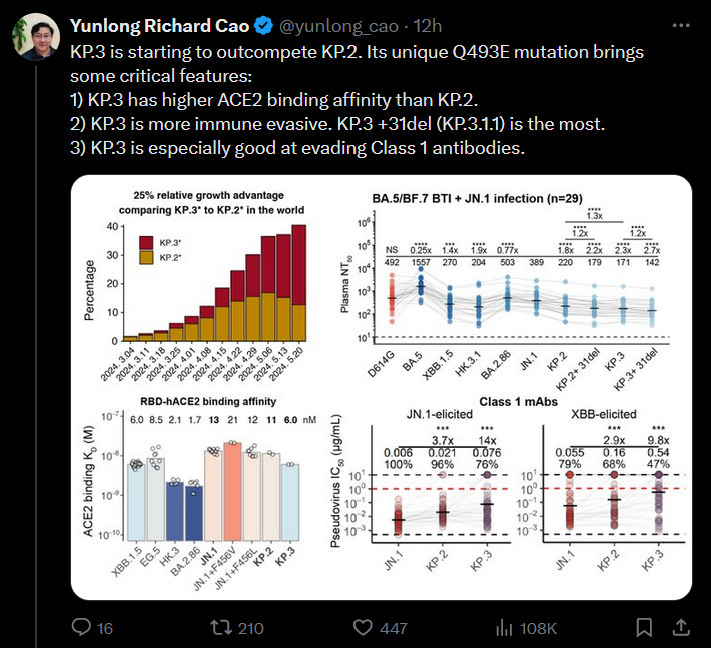
As I came to print, a beloved scientist published data on KP.3 and since I cited him earlier, I should talk about it…
It’s building concerns on TwiX that KP.2 or 3 should be the targeted variant.
But really this changes the larger equation and we need to stop trying to fit COVID into the box of how we handled regulatory decisions before it existed.
If we are going to try to live with it instead of mitigating it, then there will be consequences not only for our bodies but for regulatory process as well.
I actually went into this presentation trying to prove that we should use JN.1 for Novavax and split the antigen so that mRNA would use a KP.2 or KP.3… and they could still do that.
But we already covered what little genomic data we have and while writing this I couldn’t find a solid argument for targeting KP.2 or 3, only an argument against, when looking at the whole picture.
Worldwide KP.2 and KP.3 are trading dominance… but they also did this at an unreasonable pace.
So, the time it would take to produce new vaccines, not just Novavax but mRNA too, will inevitably mean we will be dealing with the next dominant variant that we can’t see yet. That’s actually already the case just based on the delay of the normal rollout itself.
I think it’s important to focus on the language surrounding KP.3 as a solution which imagines it as a single booster. Yes, having VRBPAC meet twice a year to update vaccines for a “booster” is actually a good idea… but it won’t be targeting KP.2 or 3 anymore.
Right now, we really need to focus on resetting everyone’s memory response via imprinting. Based on the available data, JN.1 is the right position to do that from…
And in six months, VRBPAC should be meeting again to decide what is best to boost on top of the JN.1 memory response. This variant trend shows that what we really need to be doing is pushing for twice a year variant selection meetings.
This is one of the hiccups of trying to live with COVID instead of mitigating it.
As we wrote in the final presentation slide, the FDA and CDC should be meeting twice a year to discuss variant updates… mRNA will almost always have to update and Novavax should present data on whether simply an additional shot or an antigen update would be best.
Generally speaking, Novavax has been pretty honest about the state of its vaccine, even releasing data demonstrating a memory response issue between XBB and JN.1. Luckily, that was based on three XBB shots with no wild-type immune base to start, so it’s not really applicable directly to the public.
So, the solution, from my perspective, is still two JN.1 as fast as possible, and VRBPAC should be meeting sooner to discuss variant updates. The reality is that aiming ahead of ourselves because VRBPAC doesn’t meet more often is not a great strategy.
I hope that helps.
Update: as of a few mins ago the FDA has recommended JN.1 as the primary vaccine and there was discussion of the structural changes I was asking for including greater access to anyone without using the immuncompromised status pathway.
They made it clear that they did not make the decision for JN.1 to benefit Novavax but rather because it is extremely unclear where we are headed and JN.1 is close enough until we know better. This conversation including meeting to discuss variants more often.













Part of the reason for vaccine uptake dropping is that CDC does not allow healthy people, under 65 to get any more shots since autumn of 2023. The proposed recommendation of "2 shots 2 months apart and additional shots... [within] the 6-9 month window" doesn't specify how many additional shots, and if any after this initial course is over. Looking forward to a clearly stated schedule from the CDC when the new formula arrives. Thank you for this important work you and your colleagues do.
Several of them also commented that they thought it is important to have a non-mRNA vaccine available at the same time as mRNA vaccines for people who wish to take non-mRNA vaccines.