Novavax Call to Action: Less Than Two Days to Act on First Steps.
Immediate action is followed by a multi-pronged strategy for the next few weeks.
We have very little time to make a complex maneuver.
There are just a few days until the first steps and weeks for the following steps.
If you want clarity on where we are, here is a clip from our show, Brace for Impact, with a direct quote from Novavax’s investor call.
You can also cut through all that garbage from the WSJ by reading this accurate article on where Novavax actually is. Spoilers: It’s not as bad as they made it out to be.
We need to move fast, and our moves now are critical.
What happens with these earlier moves will decide the effectiveness of our later actions. We can do it, but I have to cover some of the *eyeroll* old stuff first.
I hate writing this part of the article, where I try to prove why you should listen to me by demonstrating all our work. At this point, there is so much material that simply explaining it creates a text wall as long as most other writers’ articles alone.
This is what you need to know about us…
I, and now we, have been working on Novavax access for four years. During that time, our group's actions have played a pivotal role in ensuring vaccine access worldwide.
Or at least protecting whatever we could hold on to.
Some folks complain that I don’t post sources, but they only look at my Twitter.
I first wrote articles… These are the main ones.
Amidst this, I started doing a show multiple times a week in early 2023. We then formed a group and consistently did the show to push direct action.
This led to presentations and direct contact with members for further action…
VRBPAC Meeting: June 15th, 2023 - COVID
VRBPAC Meeting: March 5th, 2024 - Influenza.
VRBPAC Meeting: June 5th, 2024 - COVID
VRBPAC Meeting: Sept. 20th, 2025 - Pertussis
The COVID presentations were based on an “inside/outside” strategy. We did the presentation “inside” the meeting, but also had the public contact from the “outside” with not only public comments but direct contact via academic emails to members.
If you are unclear, VRBPAC is a committee of the FDA and, with that, the HHS.
Our work has been cited in a few articles.
This has not been limited to Novavax; it has encompassed all types of public health pushes for better quality safety measures across the board.
We’ve been lucky to have more success than other groups.
And we need to do it again.
This is where I say some hokey crap like “we need your help to do it…”… but we don’t.
If you want to help, that’s fantastic, but we already have enough people; while we could use more, we have enough.
If you are already convinced, great; if you aren’t, I won’t be trying anymore.
Not here at least.
I have an extremely detailed article coming up; you’ll just have to wait for that.
So, here’s the deal.
We have a bunch of things happening at once, and they are moving very quickly.
As in, right now, quickly.
After months of silence and cancelled meetings, we finally got our VRBPAC meeting to pick COVID variants for later this year.
Important note: This means we will have updated vaccines this year, or at least the process is starting. Influenza vaccines were settled on earlier this year, so let’s put that fear aside. However, it’s looking like an “updated” vaccine might not be what this moment calls for.
Typically, we would have at least a month's warning. However, because of public pressure, this meeting was only dropped on us last week, and they have decided to try to make our work less effective by playing games with how public comment works.
We can submit public comments until the 23rd, the day after the meeting.
However, you only have until May 14th at noon Eastern time to submit public comments supporting Novavax and have it shown to the committee.
I’m releasing this on May 12th, so there’s not much time to submit comments.
You can post your public comment here → https://www.federalregister.gov/documents/2025/05/08/2025-08083/vaccines-and-related-biological-products-advisory-committee-notice-of-meeting-establishment-of-a#open-comment
(This information will all be posted again at the bottom.)
That’s less than two days to get a comment before the committee.
Did that freak you out? Good.
It definitely should… However, we already have a plan for this.
While they are limiting the comments that will be shown to committee members, we have those members’ emails and will contact them directly.
So, this won’t stop us much… that all being said, comment right now.
The basic demands for the FDA are as follows…
These were our earlier demands, which have evolved a bit over time.
As we get closer to the meeting, this will change slightly.
These demands will likely include permission for Novavax to stay with JN.1.
I don’t know what Novavax will do and have not communicated with them.
This is not being said out of convenience, but rather a firm grasp on the situation.
Also, the new FDA and HHS are making strange demands for placebo testing that could inhibit vaccine availability this year.
At the same time, mRNA manufacturers will likely need to update to closer variants as they don’t have the same range as Novavax, which puts them in a tough spot.
For the record, placebo testing is required to prove that treatments work. The multi-phase trials process for vaccines is already designed to demonstrate effectiveness.
While their interpretations could use work, generally, this process works well.
Vaccines, or biologics, use a process different from traditional pharmaceutical products, but our new glorious leaders don’t seem to understand this basic concept.
Ironically, the antivaxxers are the control every year, proving the effectiveness of even mRNA.
The bar for treatments and vaccines is extremely low because until recently, that has been our limitation, but because of Operation Warp Speed, that’s all changed.
So, the place we speak to as success might feel lower than it should, and it has been at times. However, this testing has been done, and Novavax specifically has placebo testing. So, while this shouldn’t be limiting for us, they will just move the bar again.
Novavax may present data showing they can make a new vaccine fine, and I don’t doubt they are capable of it, but we likely don’t need it.
I’ve said at multiple meetings that Novavax only requires updates every two years… and Yunlong Cao of the Sato lab has said that antibodies last for about two years.
Sticking with an already heavily tested JN.1 when the vast majority, if not all, variants are JN.1 makes the most sense even without these unethical demands.
Choosing a closer variant is especially challenging because of new developments.
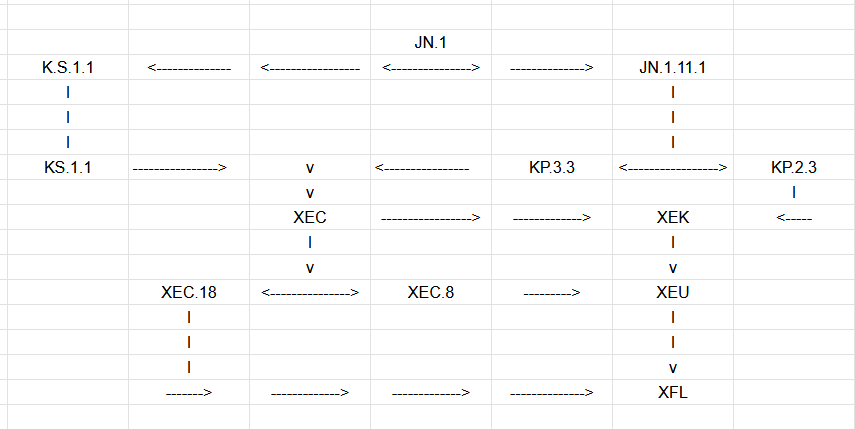
We have an XBB + JN.1 recombinant, NB.1.8.1, making a lot of noise out of Hong Kong that is now hitting the ground in California.
Technically, it’s an XBB recombinant that creates XDE, which then creates a recombinant with JN.1 to create XDV, which among its subvariants is NB.1.8.1.
So, not confusing at all.
However, it’s important to remember that the XBB recombinant is likely a persistent variant that recombined with an active JN.1 infection that is only now landing in the US, but it only has JN.1 variants to meet.
It likely being a persistent variant is important because there is very little XBB around.
Future recombinant processes will likely occur with more JN.1 subvariants, moving it closer to JN.1 overall and within the range of multiple vaccines.
This process has been steadily happening, with charts like these showing up.
The takeaway is that they are all JN.1 subvariants acting like they live in the panhandle. While that can be confusing, it can be more easily explained linearly.
I spent way longer on this than I want to admit…
It’s a little less scary when you look at it that way.
Typically, we would think variants grow out. Instead, this is a lot of “cousin relations.”
When we would typically talk about targeting the “trunk of the tree” instead of some random leaf or branch, like KP.2, this is quickly becoming more of a stump.
The point is that we can expect many more recombinants as we allow infections to run rampant, which creates a rare, predictable moment for variants.
In the same way all variants are JN.1; the same could be said about many of the currently evolving variants being XEC. We see things like XFL, but it’s just a product of multiple XEC subvariants all “inbreeding,” which is still under the JN.1 umbrella.
It’s important to note that the jump from XBB.1.5 to JN.1 was the most significant antigenic jump in the pandemic. At this stage, further recombinants will likely bring them closer together, not further apart.
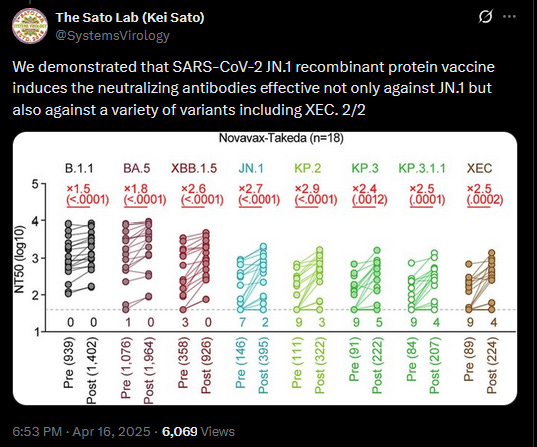
We have substantial testing showing JN.1 Novavax still works on all variants.
With an even improved response toward the newest ones.
These results show a response for XEC that is EXTREMELY close to JN.1.
This was demonstrated again by one of the top antibody labs in the world.
Also, independently of Novavax… Don’t forget to look at the XBB response, because even with the largest antigenic jump ever, it still covers it going backward, even though the XBB vaccine had trouble going forward to JN.1.
The Sato Lab’s work shows up at everyone’s meetings because it’s the best.
And, to be clear, these results were achieved with a single shot of JN.1.
Notably, it shows a high response against XBB.1.5 variants, which means it should show high effectiveness against the mutant recombinant variant on its way.
One of these new crossover variants will likely be next year's variants for Novavax. In the same way we saw BA.2.86 coming when we were picking XBB.1.5, we needed to wait for BA.2.86.1 to evolve, which we now call JN.1.
However, this did not occur on a seasonal cycle, because COVID does not need the benefit of reduced immune systems during cold weather to proliferate.
Bottom line? COVID is a year-round problem, especially if folks want to claim it’s endemic inappropriately.
The decision to change variants should be made earlier next year.
We should move the variant meeting to March and plan variant shifts in early August to meet the demands created by the start of school.
Plus, with this platform, they can pivot variants with a single shot.
Knowing that it can do this with a single shot is significant, considering we have not even tapped the potential for Novavax to reach even more variants.
But how does this work?
A benefit of Novavax is that each shot increases the range of your existing antibodies.
I can’t make it clear enough that the benefits of the products are perfectly tuned for this moment. Each shot brings your antibodies closer to evolving variants.
So, whether you have received Novavax or need to start, you’re in a good spot.
People who need protection this year break down into two groups…
Those who have already received a Novavax priming series.
Those who have not.
Unlike mRNA, those who have received Novavax would only need a single booster and would build on their previous vaccinations.
Those who have not received a full series of Novavax should start over with a full series of three shots. The first two should be eight weeks apart, and the third should be six months after the second. This has been well tested at this point.
Getting at least two is the goal, but three is better… and that data is coming up.
As JN.1 can be ready to go immediately, it will give people plenty of time to adequately vaccinate before next season. They will also need fewer shots in the long run while retaining greater protection in the process.
This multi-shot mechanism is responsible for two things we have seen with Novavax.
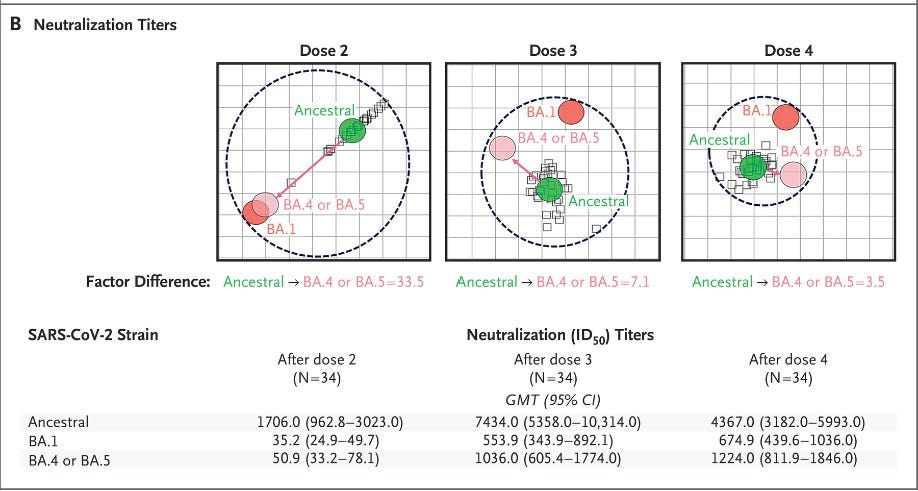
This effect has already allowed the original prototype vaccine to maintain efficacy against Omicron simply by using a third shot. (This is well proven; Novavax measured better in every way, so much that I question why we use mRNA at all.)
This is also the mechanism behind multiple shots helping with persistent virus. (This is not yet well proven, but we proved it enough to open up Novavax access two years ago in our first presentation.)
My theory for persistent virus, which we used to seek timing approval, is that your antibodies evolve from the initial variant you are exposed to. While that variant will continue to shift during initial infection, the antibodies created at the start of your infection do not. They are locked into that initial exposure at the start of this infection.
So, a variant, which is rarely more fit, can evolve to be just outside your antibodies' range. Not by intention, but just by the nature of how many virions are produced and how quickly. It’s the infinite monkeys and typewriters theorem in the real world.
COVID produces an overwhelming amount of copies, and it does it poorly.
So, it’s bound to create at least a single virion with immune evasion, but they should be very close to each other… just outside the antibodies’ reach.
Over time, they will get further apart, making your antibodies even less effective.
This is why, mechanically, increasing the range of your antibodies will assist your immune system in identifying and removing persistent viruses. Also, this antigenic range will likely increase over time, which is why speed plays a factor.
Even though persistence is not the only driver of Long COVID, it is a primary driver, and it needs to be dealt with first before we can even tell what other symptoms are left. While multiple Novavax will be and has been a solution for some people, it will not be a solution for ALL people.
But I digress, let’s stay on vaccines…
Last year, we successfully pushed for vaccines to be released earlier, and we need COVID vaccine recommendations to happen even earlier in general.

The availability should align with the start of the school year, and a vaccine with more robust protection will work continuously through the winter season.
COVID is not a seasonal virus and requires year-round protection.
Novavax achieves more robust year-round protection if folks get at least two.
This chart also means that the response never wanes to zero if you get two shots.
That is testing to 400 days after it has already plateaued for 200 days. This is why I say that folks need fewer shots: You are maintaining that baseline.
Other available COVID vaccines have not achieved this.
The end of Novavax’s response has never been captured in any study.
That means everyone needs access to a new priming series without jumping through the “immunocompromised hoop.” In previous years, they created special access for folks with Long COVID to access more shots to fight persistence, but as Long COVID is not a clearly defined term, they used immunocompromised instead.
This also opens up access for many more people.
But, back to the shots…
It has an even more robust response with three shots; this actually shows four.
So, this is not a one-time trick; it maintains between continuous shots.
While some claim that Novavax and mRNA have the same response, that’s only when measuring two mRNA against two Novavax. I have a pretty intense breakdown comparison in the larger article coming up, but it is demonstrated above.
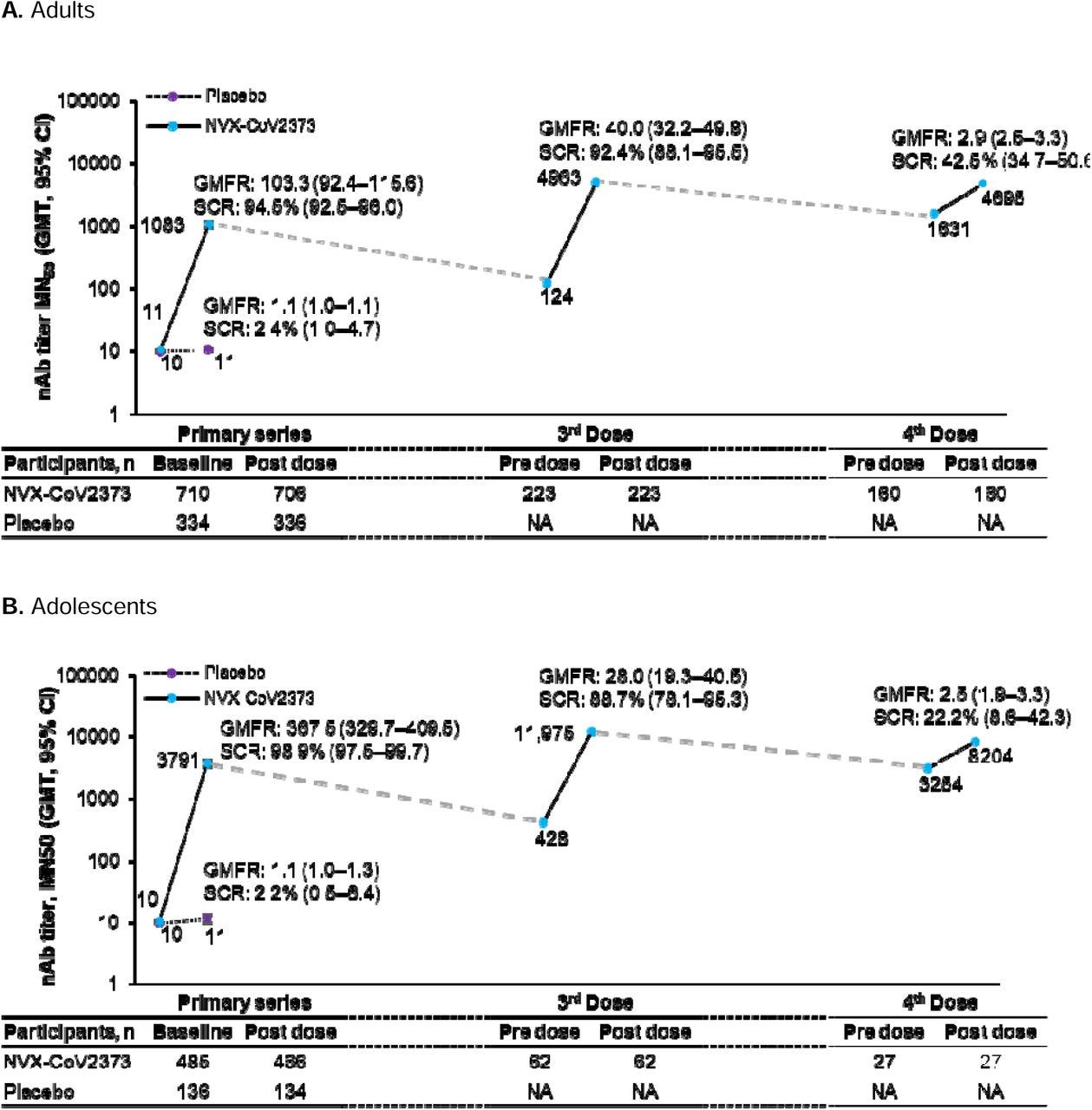
mRNA cannot achieve that response and goes no further than the initial first increase… Novavax then goes on to achieve 4- 5x that and maintains it. Let’s also note that it’s wildly effective in adolescents, specifically with an even higher response than adults.
So, Novavax and mRNA are not the same protective levels…
Novavax is 4 -5x better, has fewer AE, pivots immune memory with one shot, improves other aspects of your immune system… It’s better by every marker.
But how does this affect where we are now?
mRNA does not meet the protective levels to claim it is protective because it does not protect from the long-term effects, only short-term effects.
And you are welcome to fight with me on this, but we got that information from the FDA and CDC, and the people we are fighting have access to that data now.
There is no point in trying to misrepresent what’s going on here…
This is important because while mRNA does not meet protective levels for these characters to claim a recommendation should be allowed, Novavax is far superior in its protective levels and does meet the requirements they have put forward.
As much as they claim COVID is not harmful to children, current data points do not support that. They seem very stuck in an early 2021 mindset that has no place in the current conversation, probably also why they keep going on about mandates.
Recommendations don’t mean mandates; we just want insurance to cover the shots.
However, maintaining the idea that mRNA and Novavax have the same protective levels could only result in losing both. Novavax meets their demands, but not mRNA.
And I understand this is a tough bridge to get folks to cross.
So, while we don’t need to convince new folks, we do need to reach all the convinced ones. Whatever you can do to get the word out, do it.
If you disagree with anything I’ve said here, please push what you agree with. I’ll provide a detailed breakdown at the end of each step.
Luckily, even though the public comment that will be given to the committee closes on the 14th, we still have a week to contact members directly. So, there’s still time for folks to get their mind right on whether to send in a comment.
First is public comment, then we contact members directly until the meeting.
But it doesn’t stop there… RFK Jr. appointed Vinay Prasad, who makes wild claims about how COVID does not harm kids, vaccines are ineffective, requiring unethical placebo trials for vaccines, and other wrong and awful statements, to lead CBER, which is essentially in charge of all vaccine approvals.
He’s not only a eugenicist but also callous and evil about it, so trying to reach him other than by a brute force-style attack on all forms of communication will be unlikely to be effective. Ironically, not too different from the previous head of CBER, Marks.
However, that doesn’t mean we are without options.
RFK Jr. is on unsteady ground and is about to get grilled by the HELP committee.
So, our opportunity here is to work with the HELP committee to pressure RFK over Prasad's appointment. We might as well throw in this Surgeon General they are trying to appoint, too.
That means we need to send the HELP committee as much ammunition as possible because they may not be aware of Prasad's awful statements, and there are a lot.
This is not a person who is quietly evil; he has a podcast where he’s wildly cruel.
Here’s a page dedicated to refuting the terrible things he’s said.
Like I said, RFK Jr. is already on thin ice.
Among many of his promises was that he would not limit or eliminate vaccines. His appointment of not only Beth Hoeg, a notorious anti-vaxxer, but also a second, even worse one, as the leader of vaccines in general at CBER is not the sign he should be sending to the Senators.
But approving Novavax for both adults and kids would go a long way to prove otherwise.
The simple fact is that just like how his call for placebo testing on vaccines is unethical, it is also unethical not to allow access to beneficial vaccines.
This is where we are with Novavax, considering Novavax's expansion is just that: an expansion of usage for the existing product.
Kids and Adults use the exact same vaccine, and other countries are already opening up access. Even if you don’t want to agree with the evidence showing Novavax is better, we can at least agree that it is comparable, and as many folks cannot take mRNA, the same is true of kids. We need a non-mRNA alternative for children, too.
This is an ongoing accessibility issue. The current stance of the federal government is unethical when you consider the best vaccine for kids, or even the only vaccine certain kids can get, is just sitting on the shelves going to waste while they are unprotected.
Kids who need an mRNA alternative are likely to need these vaccines the most.
… just a quick sidebar before we get to the action plan.
There will be some debate about whether what I’m saying is totally ridiculous, with mRNA advocates talking about how this or that variant is the best choice.
And that was fun in previous years, but this year it hits a bit differently.
We already have a glimmer of the plan for mRNA’s future, which is a “second-gen vaccine,” but really, it’s just the old bivalent, but now with less antigen.
This was the only COVID vaccine asked to present at the last rushed ACIP meeting.
What’s funny, but also not funny, about this is that they significantly reduced the antigen, but this did not lead to an equal reduction in Adverse Events.
That tip there with the stripes? Those are Grade 3 Adverse Events.
If Novavax had a consistent Grade 3 response, we would never have made it here.
The bivalent failed rather famously, and the proof is in how quickly we moved back to monovalent vaccines. The data was clear then, and the data is clear now.
You can look at the entire slide deck here, but when mRNA advocates try to give us a hard time for suggesting we stick with JN.1… Remember, they plan to go back to the bivalent… and good luck trying to figure that mess out.
Trying to pick a single variant is hard enough, but two?!?! That’s a lot of money riding on not only one but TWO games of Pin the Tail on the Donkey.
So, if they try to give you any trouble, just remind them that’s their position already.
We cannot, under any circumstances, let them move us back to bivalent COVID vaccines. Will we do multi-valent-style “combo” vaccines, with COVID and influenza? Yeah, probably, and that’s the “multi-valent” style vaccine RFK Jr. would mean in a world where people are prepared to do their jobs in the best interests of the people.
Unfortunately, we are not in that world… Not yet, at least.
Here’s a direct breakdown of actions:
First, all contact should be friendly and polite. This push does not support hating on or attacking these people through this process. That will not help.
Step 1
First: VRBPAC public comment & Contact Senate HELP members.
Deadline: Both are on May 14th, noon Eastern.
VRBPAC
VRBPAC demands:
Previous Demands
Immediate approval of Novavax’s BLA
Streamline approval of pediatric expansion
Expand the expiration date
New Demands
Move COVID variant selection to early in the year to align release with the school year, treating COVID as a year-round virus instead of a seasonal one. With greater protection from Novavax, it will last year-round instead of a few months.
Choosing JN.1 for Novavax is a very reasonable choice; it not only circumvents many of the roadblocks being erected by new leadership but is also a safe choice. Choosing JN.1 will also give adequate space to change approval timing next year.
Recommend a new priming series be available when switching to Novavax; the immunocompromised rules have proven effective, but this is the best way to use this product. This is the protection available, and folks should have access.
Clarity on changes to the Pediatric Vaccine Application made recently. Novavax reported changes to testing for the supplemental to their BLA for pediatric expansion. However, it’s unclear what those changes are.
Under no circumstances should we recommend moving back to bivalent COVID vaccines; these failed miserably last time and would be a huge step back.
VRBPAC Public Comment Link → https://www.regulations.gov/commenton/FDA-2025-N-1146-0001
You can also send public comment directly through the federal register → https://www.federalregister.gov/documents/2025/05/08/2025-08083/vaccines-and-related-biological-products-advisory-committee-notice-of-meeting-establishment-of-a#open-comment
I’m 90% sure they all go to the same place, and regulations.gov is friendlier.
We will follow up with direct contact after this next step.
Senate HELP Committee & HHS
Deadline: May 14th
HELP Committee Demands: (there are more questions we want asked)
Specifically, the hiring of Vinay Prasad and the current Surgeon General nominee.
Hold up on Novavax approval; it has already been delayed a month and a half.
COVID variant hearings were only held after immense public pressure.
Why have there been changes to approval for pediatric vaccines at the FDA?
Does he plan to limit vaccines or not approve new ones?
Does he think COVID is dangerous for children?
Will he approve the Novavax expansion for children?
Does he believe Long COVID is real?
Are we studying Monoclonal Antibodies and infusion antivirals for LC?
Does his concern for chronic illness include those caused by airborne pathogens?
HELP Senate Committee Members:
Bernie Sanders (I-VT) (202) 224-5141
Patty Murray (D-WA) (202) 224-2621
Tammy Baldwin (D-WI) (202) 224-5653
Christopher Murphy (D-CT) (202) 224-4041
Tim Kaine (D-VA) (202) 224-4024
Maggie Hassan (D-NH) (202) 224-3324
John W. Hickenlooper (D-CO) (202) 224-5941
Ed Markey (D-MA) (202) 224-2742
Andy Kim (D-NJ) (202) 224-4744
Lisa Blunt Rochester (D-DE) (202) 224-2441
Angela D. Alsobrooks (D-MD) (202) 224-4524
Bill Cassidy (R-LA) (202) 224-5824
Rand Paul (R-KY) (202) 224-4343
Susan M. Collins (R-ME) (202) 224-2523
Lisa Murkowski (R-AK) (202) 224-6665
Markwayne Mullin (R-OK) (202) 224-4721
Roger Marshall (R-KS) (202) 224-4774
Tim Scott (R-SC) (202) 224-6121
Josh Hawley (R-MO) (202) 224-6154
Tommy Tuberville (R-AL) (202) 224-4124
Jim Banks (R-IN) (202) 224-4814
Jon Husted (R-OH) (202)-224-3353
Ashley Moody (R-FL) (202) 224-3041
HHS
When you are done contacting those Senators, contact the HHS.
Use the same or similar talking points.
Contacts: 1-877-696-6775
More TBD
Step 2
Contacting VRBPAC and ACIP members.
VRBPAC
Deadline: May 22 for final public comment and direct contact with members.
VRBPAC Demands: (same as before)
Previous Demands
Immediate approval of Novavax’s BLA
Streamline approval of pediatric expansion
Expand the expiration date
New Demands
Move COVID variant selection to early in the year to align release with the school year, treating COVID as a year-round virus instead of a seasonal one. With greater protection from Novavax, it will last year-round instead of a few months.
Choosing JN.1 for Novavax is a very reasonable choice; it circumvents many of the roadblocks being erected by new leadership and is also a safe choice. Choosing JN.1 will also give adequate space to change approval timing next year.
Recommend a new priming series be available when switching to Novavax; the immunocompromised rules have proven effective, but this is the best way to use this product. This is the protection available, and folks should have access.
Clarity on changes to the Pediatric Vaccine Application made recently. Novavax reported changes to testing for the supplemental to their BLA for pediatric expansion. However, it’s unclear what those changes are.
Under no circumstances should we recommend moving back to bivalent COVID vaccines; these failed miserably last time and would be a huge step back.
FDA - VRBPAC voting members for contact
• Hana M. El Sahly: hanae@bcm.edu
• Adam C. Berger: adam.berger@nih.gov
• Henry H. Bernstein: hbernstein@northwell.edu
• Archana Chatterjee: archana.chatterjee@rosalindfranklin.edu
• (New) Anna Durbin: adurbin1@jhu.edu
• Hayley Gans: hagans@stanford.edu
• Robert S. Janssen: N/A
• Luis Jódar: luis.jodar@pfizer.com ← wtf @pfizer
• CAPT Sarah Meyer: vif6@cdc.gov
• Arnold S. Monto: asmonto@umich.edu
• (New) Flor M. Munoz-Rivas: florm@bcm.edu
• Michael R. Nelson: mrn8d@virginia.edu
• Paul A. Offit: Not him
• (NEW) Saad B. Ome: saad.omer@yale.edu
• Stanley M. Perlman: stanley-perlman@uiowa.edu
• Jay M. Portnoy: jportnoy@cmh.edu
• Eric J. Rubin: erubin@hsph.harvard.edu
All three new members specifically have an expertise in pediatrics.
ACIP
Deadline: ACIP Event date June 25th- 26th.
We will follow up with the ACIP public comment when it opens.
ACIP Demands:
Activists fought hard for the COVID vaccine recommendations with earlier ACIP committees. Please do not erase that work by removing the recommendation. The recommendation exists primarily to require insurance companies to cover them at no cost; losing this would be very expensive.
Recommend a new priming series for people switching to Novavax so that it is easily available. If folks want access, they should have access.
Recommend pediatric Novavax expansion so we can have a pediatric mRNA alternative. Some folks cannot get mRNA, and they often need a COVID vaccine the most. They need an alternative; it is unethical to do otherwise when the vaccine is immediately available. It is called an expansion because it expands usage to include pediatric use; other countries are ahead of us on this.
Clarity on changes to the Pediatric Vaccine Application made recently.
Under no circumstances should we recommend moving back to bivalent COVID vaccines; these failed miserably last time and would be a huge step back.
CDC - ACIP voting Members for contact
• Chair: Helen Keipp Talbot: keipp.talbot@vumc.org
• Edwin Jose Asturias: edwin.asturias@ucdenver.edu
• Noel T. Brewer: ntb@unc.edu
• Oliver Brooks: olibro@aol.com
• Lin H. Chen: lchen@hms.harvard.edu
• Helen Y. Chu: helenchu@uw.edu
• Sybil Cineas: Sybil Cineas@brown.edu
• Denise J Jamieson: denise-jamieson@uiowa.edu
• Mini Kamboj: kambojm@mskcc.org
• George Kuchel: kuchel@uchc.edu
• Jamie Loehr: Dr.Jamie.Loehr@Gmail.com
• Yvonne Maldonado: bonniem@stanford.edu
• Charlotte A. Moser: moser@email.chop.edu
• Robert Schechter: R.Schechter@cdph.ca.gov
• Albert C Shaw: albert.shaw@yale.edu
• Karyn Lyons: Karyn.Lyons@Illinois.gov
• Jane Zucker: jzucker@health.nyc.gov
If you would like to follow any of my socials, not recommended, or make a donation, definitely recommended… then the links are in the button below.
Also, here is a quick plug for our show Brace for Impact. We are currently on Twitter/X spaces on Tuesdays and Thursdays around 6:00 pm PST, but the time is not set in stone.
You’re also welcome to join our Discord, but be prepared for a small test to enter.












Good luck!!!!
On the drop down list they as you to choose a category that describes what your comment is about and then there a long alphabetical list from Academia to State Government
What category do we use?