Action Needed: Demand The CDC Remove Limits On COVID Vaccine Access & ACIP Public Comment Closes Tomorrow The 18th at 11:59 pm ET
America rolled out a 'once a year' plan that failed miserably. We need to remove the restrictions that failed policy created.
For anyone who wants access to Novavax, there is a very important vote happening that could decide how easily you can access your additional doses to start over for the maximum real world effectiveness of the product.
This has a very short timetable, with public comment closing on the 18th at 11:59 pm.
Demand: The CDC needs to change their recommendation to include two shots a year, with a third available if needed FOR ALL PEOPLE, with additional shots available for the immunocompromised.
Link→ https://www.regulations.gov/commenton/CDC-2024-0072-0001
If you are reading this in your email then that’s tomorrow.
We also have a secondary call to action on the way after public comment closes...
Our focus right now though, is a very specific call to action so that folks can access not just Novavax but all COVID vaccines.
Some have said COVID vaccines are only needed once a year, others think twice is enough, but these limits are confusing and put pressure on pharmacists and patients alike.
But after fighting for Novavax access for two years…
We are looking at potentially a major endpoint.
All you folks who are looking to get your second dose in a few weeks…
I’m looking at you.
There is some debate on whether two Novavax doses is necessary when switching from mRNA… And this debate is very reasonable.
Though it is widely accepted that at least two shots of any vaccine is required for any lasting response.
And it has recently come to light that mRNA does not create the desired persistent antibody response that we would normally expect from a priming series.
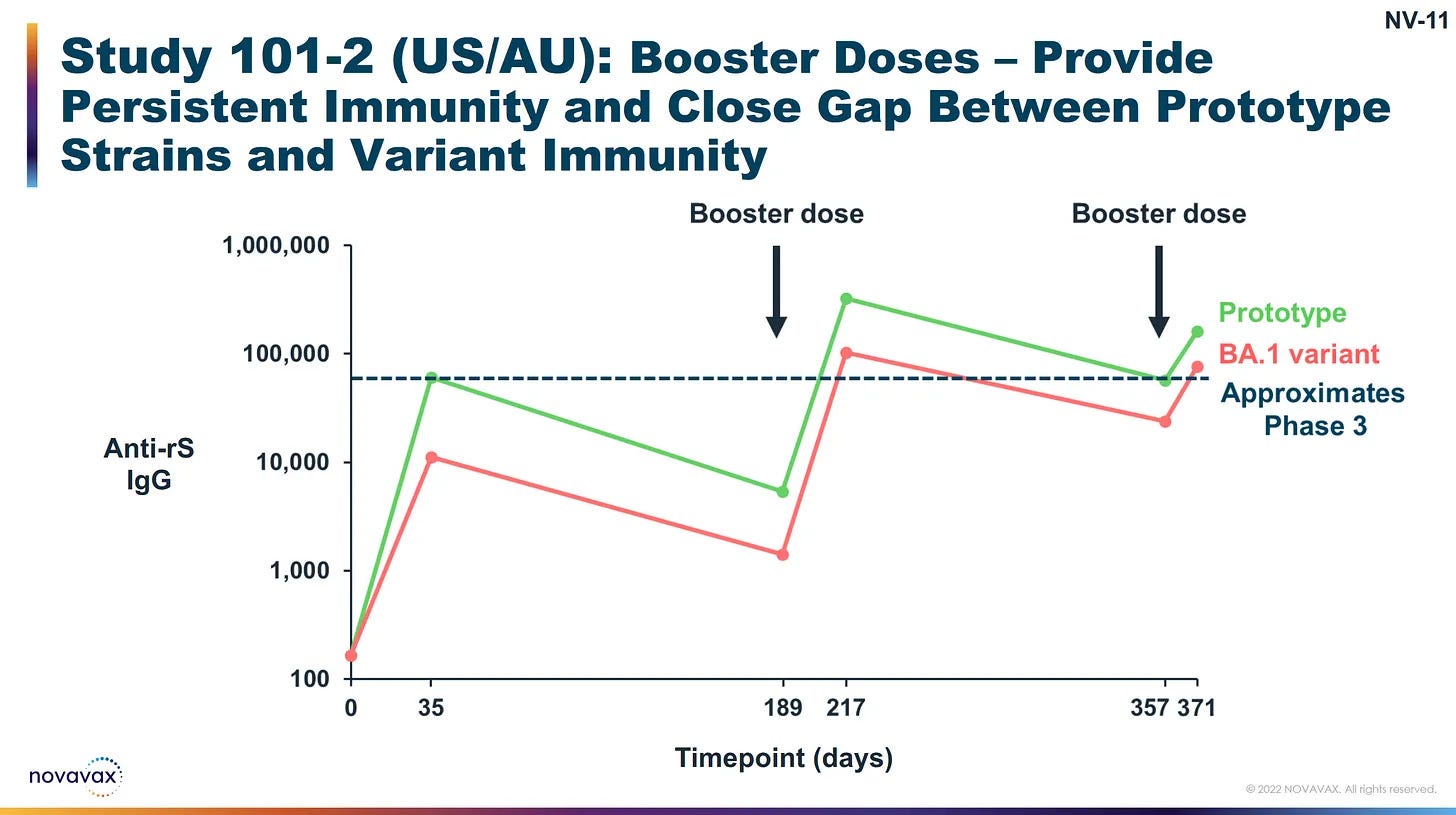
While we do see that Novavax does create the desired result for at least 10 months, which was the period of testing… So, not it’s limit, just what was tested…
I can still understand why we might need to debate these ideas but…
What I’m sure we can all agree on is that we SHOULD be able to access them if it is agreed to be necessary.
Now, I am working on a more extensive explanation for why priming again with new versions of vaccines is a good idea, even needed, but I simply won’t finish it in time for this call to action…
It will be done before the meeting, but after public comment closes.
So, some folks might need some time to decide if getting a new priming series (formally known as a primary series) is necessary for them…
However, the time to decide if we CAN is right now.
While the FDA decides if a vaccine is safe or not to use, the CDC decides how said vaccines should be used properly.
On October 23rd, the Advisory Committee on Immunization Practices (ACIP), a CDC committee, is meeting to decide who can access additional doses of COVID vaccines.
They are voting on a few things, but here is the voting language for this meeting:
Now, it’s likely that they will vote yes on this, generally speaking…
And that’s a good thing.
It was made clear at FDA’s VRBPAC committee meeting earlier this year that the “immunocompromised route” was set up to allow anyone access to additional doses, though this does create a bit of a “hoop” folks have to jump through.
The way it’s set up now is that you can get the additional doses required to complete your Novavax series if you claim to be immunocompromised, and they are barred from asking for proof… At that same VRBPAC, it was explained that this route was essentially a “middle ground” on making doses available without officially recommending it.
But this puts a lot of stress on a lot of things that don’t need that stress.
So, instead of being at risk of losing ground, we have the opportunity to push forward and remove all the hoops to allow for more general access.
It’s even likely that the FDA and CDC will approve an additional shot early next year for the non-immunocompromised in preparation for a Summer wave, but a lot of people are going to need an additional shot this year before that.
And this is good because mRNA protection will have waned, and for Novavax user this will increase the breadth of their antibodies to reach newly developed variants.
But for this call to action, to put it simply…
Anyone who wants to access these vaccines should be able to access them.
It was also said at the same VRBPAC meeting that these vaccines have excellent safety profiles and with uptake low, they should be available to whoever wants them…
As long as they are at least two months apart.
The official recommendation should move to twice a year with a summer and winter wave predicted for the foreseeable future…
But there should be another shot available to anyone, should they need it.
There are a lot of reasons someone might need additional access…
When getting mRNA, they might want to update their memory response. There are examples of this type of update even being necessary with influenza as well.
We’ve learned from flu vaccines, that getting vaccinated every year without exposure can make hospitalization risk higher. We can likely mitigate that with an additional shot to update the memory response.
If folks switch to Novavax, they may want to prime with the protein-based options and that can include 3 shots; 2 shots 8 weeks apart and a 3rd no sooner than 6 months later.
They might have a high risk event to boost their immunity for and mRNA protection is know to only last for 112 days. That’s three to cover 365 days.
As the pandemic drags on, someone might go a long time between shots, making them want to start over… They should have that freedom.
And at the end of the day, ACIP approval means insurance coverage.
There are many reasons someone might need additional doses, and putting the power to decide into the hands of pharmacists is beyond the scope of their role.
Opening up general access is the way to simplify the process.
Most will only get one, some will get two, and when folks restart with Novavax, a third should be available.
But if they want to reach the maximum response, as the product was intended, three shots in the first year is required.
This response is beyond the scope of the goals of our disease control.
You might not realize it, but…
The last year or so has been a bit of an experiment…
There was an attempt to roll out a ‘once a year’ policy for access to COVID vaccines and it’s very clear that policy does not meet the demands of the moment.
Where that policy could succeed is by allowing folks to get three Novavax to complete a priming series. Then once a year will likely be sufficient until there are extreme mutational shifts, like the jump from XBB.1.5 to BA.2.86.1 (JN.1) this year.
Regarding my own personal experience, I have received only Novavax… I had 2 doses 8 weeks apart and a 3rd at 6 months. In addition to that, I have had 2 boosters, one 10 months later, and now my most recent a full year after the last.
I have not had COVID in that time.
In combination with a high quality respirator, I am confident in that protection, though this year I will be looking to get an additional shot of Novavax that will both peak my antibody response but also increase the breadth of those antibodies, so they will be effective against newly developing variants for a summer wave.
So, to recap…
I needed three shots in the first year when I started…
I waited roughly a year between the last two, but with the advanced mutations between last year and what we expect this year, I will likely want an additional one before the summer wave.
This is a more complex rotation than the CDC can normally administer on a large scale, so it will likely have to be shaped over a few years… But the focus is on the need for the initial three shots in a year to use the product as it was originally intended.
And if you already got your three to start then we have to make sure that others have that same opportunity…
Until we can develop that nuance… simple is better, we can add nuance over time.
So, right now, we need to remove the limitations the failed ‘once a year’ policy created and allow access to whoever wants these vaccines.
And as this is a fluid situation that will change over time, for this year…
That means a recommendation of two vaccines a year…
With a third available if needed…
To be approved by ACIP, but not necessarily recommended for all people.
If you need more, then you should be working with a doctor.
This way insurance will cover the shots, and pharmacists don’t have to decide.
COVID is not a seasonal virus, folks need year-round protection.
Until we improve our intramuscular vaccines or develop intranasals…
This is what the moment requires.
Because according to the CDC, the people who run this committee, everyone is at risk of severe illness.
So, who better to advise them, than themselves?
The public comment is currently up to to almost 1500 between comments accepted and comments they have received.
Kudos to the People’s CDC for making most of that happen. I really want to thank them for using their space to draw attention to this cause.
But their ask for two a year simply does not go far enough to be inclusive to all groups… Especially if you are restarting with Novavax for the maximum effect.
Or even if you want complete year-round protection from mRNA.
To actually protect people, we need to go just that little bit further.
Link—→ https://www.regulations.gov/commenton/CDC-2024-0072-0001
Deadline is Oct 18th, that’s tomorrow before 11:59 PM eastern.
DO NOT WAIT ON THIS!
Demand: The CDC needs to change their recommendation to include two shots a year, with a third available if needed FOR ALL PEOPLE, with additional shots available for the immunocompromised.
Remember, if you already made a comment, there’s nothing stopping you from making a second one.
After public comment closes on the 18th, we’re going to dust off a secondary method to contact committee members.
This is not a moment for proper etiquette, though we should still be polite.
If you read all this and are a bit confused why you should listen to me on this, know that this is part of a greater action to create change at the FDA and CDC while making us safer at the same time.
If you like to take a moment to review our previous presentations to see if our work deserves your attention…
Here’s the presentation we made for the June 15, 2023 meeting for wider Novavax approval.
Then after that we made a presentation on influenza vaccines on March 5, 2024.
And on June 5th, 2024 we presented for the updated strain selection and expanding Novavax access.
And last our Pertussis presentation on Sept. 20th, 2024.
If you would like to get more involved on a regular basis, then you have a few options:







oh fab, this showed up in my feed now (the 19th) 🙃
Comment Tracking Number m2e-nbn1-lmhr
I reminded them that their decisions impact policy world wide and to keep that in mind, that they should keep other vaccines in the mix as the virus evolves, consider that vulnerable populations will vary with economic conditions (and we're heading into bad time) and to look further into why mRNA fades faster.